Updates to standardized derived element documentation
This commit is contained in:
parent
7736b307e4
commit
7d5be9b4c6
|
|
@ -1,17 +0,0 @@
|
|||
The COHORT_ATTRIBUTE table contains attributes associated with each subject within a cohort, as defined by a given set of criteria for a duration of time. The definition of the Cohort Attribute is contained in the ATTRIBUTE_DEFINITION table.
|
||||
|
||||
Field|Required|Type|Description
|
||||
:---------------------|:--------|:------------|:------------------------------
|
||||
|cohort_definition_id|Yes|integer|A foreign key to a record in the [COHORT_DEFINITION](https://github.com/OHDSI/CommonDataModel/wiki/COHORT_DEFINITION) table containing relevant Cohort Definition information.|
|
||||
|subject_id|Yes|integer|A foreign key to the subject in the Cohort. These could be referring to records in the PERSON, PROVIDER, VISIT_OCCURRENCE table.|
|
||||
|cohort_start_date|Yes|date|The date when the Cohort Definition criteria for the Person, Provider or Visit first match.|
|
||||
|cohort_end_date|Yes|date|The date when the Cohort Definition criteria for the Person, Provider or Visit no longer match or the Cohort membership was terminated.|
|
||||
|attribute_definition_id|Yes|integer|A foreign key to a record in the [ATTRIBUTE_DEFINITION](https://github.com/OHDSI/CommonDataModel/wiki/ATTRIBUTE_DEFINITION) table containing relevant Attribute Definition information.|
|
||||
|value_as_number|No|float|The attribute result stored as a number. This is applicable to attributes where the result is expressed as a numeric value.|
|
||||
|value_as_concept_id|No|integer|The attribute result stored as a Concept ID. This is applicable to attributes where the result is expressed as a categorical value.|
|
||||
|
||||
### Conventions
|
||||
* Each record in the COHORT_ATTRIBUTE table is linked to a specific record in the COHORT table, identified by matching cohort_definition_id, subject_id, cohort_start_date and cohort_end_date fields.
|
||||
* It adds to the Cohort records calculated co-variates (for example age, BMI) or composite scales (for example Charleson index).
|
||||
* The unifying definition or feature of the Cohort Attribute is captured in the attribute_definition_id referring to a record in the ATTRIBUTE_DEFINITION table.
|
||||
* The actual result or value of the Cohort Attribute (co-variate, index value) is captured in the value_as_number (if the value is numeric) or the value_as_concept_id (if the value is a concept) fields.
|
||||
|
|
@ -11,14 +11,14 @@ Field|Required|Type|Description
|
|||
|condition_era_id|Yes|integer|A unique identifier for each Condition Era.|
|
||||
|person_id|Yes|integer|A foreign key identifier to the Person who is experiencing the Condition during the Condition Era. The demographic details of that Person are stored in the PERSON table.|
|
||||
|condition_concept_id|Yes|integer|A foreign key that refers to a standard Condition Concept identifier in the Standardized Vocabularies.|
|
||||
|condition_era_start_date|Yes|date|The start date for the Condition Era constructed from the individual instances of Condition Occurrences. It is the start date of the very first chronologically recorded instance of the condition.|
|
||||
|condition_era_end_date|Yes|date|The end date for the Condition Era constructed from the individual instances of Condition Occurrences. It is the end date of the final continuously recorded instance of the Condition.|
|
||||
|condition_era_start_datetime|Yes|date|The start date for the Condition Era constructed from the individual instances of Condition Occurrences. It is the start date of the very first chronologically recorded instance of the condition.|
|
||||
|condition_era_end_datetime|Yes|date|The end date for the Condition Era constructed from the individual instances of Condition Occurrences. It is the end date of the final continuously recorded instance of the Condition.|
|
||||
|condition_occurrence_count|No|integer|The number of individual Condition Occurrences used to construct the condition era.|
|
||||
|
||||
### Conventions
|
||||
* Condition Era records will be derived from the records in the CONDITION_OCCURRENCE table using a standardized algorithm.
|
||||
* Each Condition Era corresponds to one or many Condition Occurrence records that form a continuous interval.
|
||||
* The condition_concept_id field contains Concepts that are identical to those of the CONDITION_OCCURRENCE table records that make up the Condition Era. In contrast to Drug Eras, Condition Eras are not aggregated to contain Conditions of different hierarchical layers.
|
||||
* The Condition Era Start Date is the start date of the first Condition Occurrence.
|
||||
* The Condition Era End Date is the end date of the last Condition Occurrence.
|
||||
* Condition Eras are built with a Persistence Window of 30 days, meaning, if no occurence of the same condition_concept_id happens within 30 days of any one occurrence, it will be considered the condition_era_end_date.
|
||||
|
||||
No.|Convention Description
|
||||
:--------|:------------------------------------
|
||||
| 1 | Condition Era records will be derived from the records in the CONDITION_OCCURRENCE table using a standardized algorithm. |
|
||||
| 2 | Each Condition Era corresponds to one or many Condition Occurrence records that form a continuous interval.<br><ul><li>The condition_concept_id field contains Concepts that are identical to those of the CONDITION_OCCURRENCE table records that make up the Condition Era. In contrast to Drug Eras, Condition Eras are not aggregated to contain Conditions of different hierarchical layers.</li><li>The Condition Era Start Date is the start date of the first Condition Occurrence.</li><li>The Condition Era End Date is the end date of the last Condition Occurrence.</li></ul>
|
||||
| 3 | Condition Eras are built with a Persistence Window of 30 days, meaning, if no occurrence of the same condition_concept_id happens within 30 days of any one occurrence, it will be considered the condition_era_end_date.
|
||||
|
|
|
|||
|
|
@ -7,18 +7,22 @@ Field|Required|Type|Description
|
|||
|drug_concept_id|Yes|integer|A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the active Ingredient Concept.|
|
||||
|unit_concept_id|Yes|integer|A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the unit concept.|
|
||||
|dose_value|Yes|float|The numeric value of the dose.|
|
||||
|dose_era_start_date|Yes|date|The start date for the drug era constructed from the individual instances of drug exposures. It is the start date of the very first chronologically recorded instance of utilization of a drug.|
|
||||
|dose_era_end_date|Yes|date|The end date for the drug era constructed from the individual instance of drug exposures. It is the end date of the final continuously recorded instance of utilization of a drug.|
|
||||
|dose_era_start_datetime|Yes|date|The start date for the drug era constructed from the individual instances of drug exposures. It is the start date of the very first chronologically recorded instance of utilization of a drug.|
|
||||
|dose_era_end_datetime|Yes|date|The end date for the drug era constructed from the individual instance of drug exposures. It is the end date of the final continuously recorded instance of utilization of a drug.|
|
||||
|
||||
### Conventions
|
||||
* Dose Eras will be derived from records in the DRUG_EXPOSURE table and the Dose information from the DRUG_STRENGTH table using a standardized algorithm.
|
||||
* Each Dose Era corresponds to one or many Drug Exposures that form a continuous interval and contain the same Drug Ingredient (active compound) at the same effective daily dose.
|
||||
* Dose Form information is not taken into account. So, if the patient changes between different formuations, or different manufacturers with the same formulation, the Dose Era is still spanning the entire time of exposure to the Ingredient.
|
||||
* The daily dose is calculated for each DRUG_EXPOSURE record by calculating the total dose of the record and dividing by the duration.
|
||||
* The total dose of a DRUG_EXPOSURE record is calculated with the help of the DRUG_STRENGTH table containing the dosage information for each drug as following:
|
||||
|
||||
No.|Convention Description
|
||||
:--------|:------------------------------------
|
||||
| 1 | Dose Eras will be derived from records in the DRUG_EXPOSURE table and the Dose information from the DRUG_STRENGTH table using a standardized algorithm. |
|
||||
| 2 | Each Dose Era corresponds to one or many Drug Exposures that form a continuous interval and contain the same Drug Ingredient (active compound) at the same effective daily dose. |
|
||||
| 3 | Dose Form information is not taken into account. So, if the patient changes between different formulations, or different manufacturers with the same formulation, the Dose Era is still spanning the entire time of exposure to the Ingredient.
|
||||
| 4 | The daily dose is calculated for each DRUG_EXPOSURE record by calculating the total dose of the record and dividing by the duration. |
|
||||
|
||||
|
||||
The total dose of a DRUG_EXPOSURE record is calculated with the help of the DRUG_STRENGTH table containing the dosage information for each drug as following:
|
||||
|
||||
|
||||
| 1 | Tablets and other fixed amount formulations |
|
||||
| 5 | Tablets and other fixed amount formulations |
|
||||
|:-----------------|:-----------------------------------------|
|
||||
||*Example: Acetaminophen (Paracetamol) 500 mg, 20 tablets.*|
|
||||
| DRUG_STRENGTH | The denominator_unit is empty |
|
||||
|
|
@ -27,14 +31,14 @@ Field|Required|Type|Description
|
|||
|`Ingredient dose=`|`quantity x amount_value [amount_unit_concept_id]`|
|
||||
||*`Acetaminophen dose = 20 x 500mg = 10,000mg`*|
|
||||
|
||||
| 2 | Puffs of an inhaler |
|
||||
| 6 | Puffs of an inhaler |
|
||||
|:-----------------|:-----------------------------------------|
|
||||
||Note: There is no difference to use case 1 besides that the DRUG_STRENGTH table may put {actuat} in the denominator unit. In this case the strength is provided in the numerator.|
|
||||
| DRUG_STRENGTH | The denominator_unit is {actuat}|
|
||||
| DRUG_EXPOSURE | The quantity refers to the number of pieces, e.g. puffs |
|
||||
| `Ingredient dose=`|`quantity x numerator_value [numerator_unit_concept_id]`|
|
||||
|
||||
| 3 | Quantified Drugs which are formulated as a concentration |
|
||||
| 7 | Quantified Drugs which are formulated as a concentration |
|
||||
|:-----------------|:-----------------------------------------|
|
||||
||*Example: The Clinical Drug is Acetaminophen 250 mg/mL in a 5mL oral suspension. The Quantified Clinical Drug would have 1250 mg / 5 ml in the DRUG_STRENGTH table. Two suspensions are dispensed.*|
|
||||
| DRUG_STRENGTH | The denominator_unit is either mg or mL. The denominator_value might be different from 1. |
|
||||
|
|
@ -43,7 +47,7 @@ Field|Required|Type|Description
|
|||
| `Ingredient dose=`|`quantity x numerator_value [numerator_unit_concept_id]`|
|
||||
||*`Acetaminophen dose = 2 x 1250mg = 2500mg`*|
|
||||
|
||||
| 4 | Drugs with the total amount provided in quantity, e.g. chemotherapeutics |
|
||||
| 8 | Drugs with the total amount provided in quantity, e.g. chemotherapeutics |
|
||||
|:-----------------|:-----------------------------------------|
|
||||
||*Example: 42799258 "Benzyl Alcohol 0.1 ML/ML / Pramoxine hydrochloride 0.01 MG/MG Topical Gel" dispensed in a 1.25oz pack.*|
|
||||
| DRUG_STRENGTH | The denominator_unit is either mg or mL.|
|
||||
|
|
@ -55,7 +59,7 @@ Field|Required|Type|Description
|
|||
||*`Pramoxine hydrochloride dose = 37 x 0.01mg x 1000 = 370mg`*|
|
||||
||*Note: The analytical side should check the denominator in the DRUG_STRENGTH table. As mg is used for the second ingredient the factor 1000 will be applied to convert between g and mg.*|
|
||||
|
||||
| 5 | Compounded drugs |
|
||||
| 9 | Compounded drugs |
|
||||
|:-----------------|:-----------------------------------------|
|
||||
||*Example: Ibuprofen 20%/Piroxicam 1% Cream, 30ml in 5ml tubes.*|
|
||||
| DRUG_STRENGTH | We need entries for the ingredients of Ibuprofen and Piroxicam, probably with an amount_value of 1 and a unit of mg.|
|
||||
|
|
@ -66,7 +70,7 @@ Field|Required|Type|Description
|
|||
||*`Piroxicam dose = 0.3 x 1mg x 1000 = 300mg`*|
|
||||
||*Note: The analytical side determines that the denominator for both ingredients in the DRUG_STRENGTH table is mg and applies the factor 1000 to convert between mL/g and mg.*|
|
||||
|
||||
| 6 | Drugs with the active ingredient released over time, e.g. patches |
|
||||
| 10 | Drugs with the active ingredient released over time, e.g. patches |
|
||||
|:-----------------|:-----------------------------------------|
|
||||
||*Example: Ethinyl Estradiol 0.000833 MG/HR / norelgestromin 0.00625 MG/HR Weekly Transdermal Patch*|
|
||||
| DRUG_STRENGTH | The denominator units refer to hour.|
|
||||
|
|
|
|||
|
|
@ -5,23 +5,23 @@ Field|Required|Type|Description
|
|||
|drug_era_id|Yes|integer|A unique identifier for each Drug Era.|
|
||||
|person_id|Yes|integer|A foreign key identifier to the Person who is subjected to the Drug during the fDrug Era. The demographic details of that Person are stored in the PERSON table.|
|
||||
|drug_concept_id|Yes|integer|A foreign key that refers to a Standard Concept identifier in the Standardized Vocabularies for the Ingredient Concept.|
|
||||
|drug_era_start_date|Yes|date|The start date for the Drug Era constructed from the individual instances of Drug Exposures. It is the start date of the very first chronologically recorded instance of conutilization of a Drug.|
|
||||
|drug_era_end_date|Yes|date|The end date for the drug era constructed from the individual instance of drug exposures. It is the end date of the final continuously recorded instance of utilization of a drug.|
|
||||
|drug_era_start_datetime|Yes|date|The start date for the Drug Era constructed from the individual instances of Drug Exposures. It is the start date of the very first chronologically recorded instance of conutilization of a Drug.|
|
||||
|drug_era_end_datetime|Yes|date|The end date for the drug era constructed from the individual instance of drug exposures. It is the end date of the final continuously recorded instance of utilization of a drug.|
|
||||
|drug_exposure_count|No|integer|The number of individual Drug Exposure occurrences used to construct the Drug Era.|
|
||||
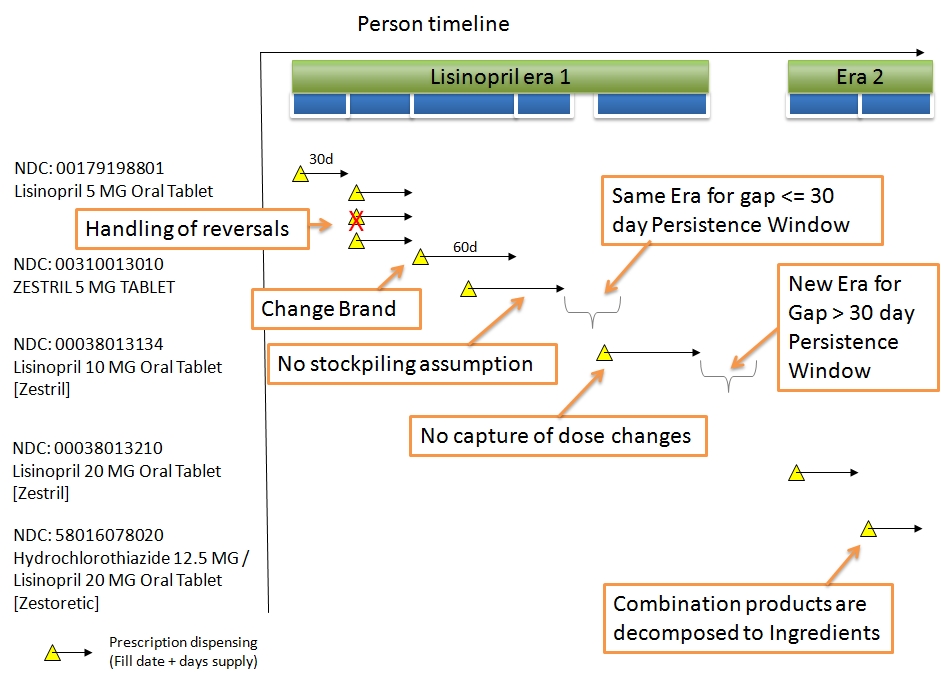
|gap_days|No|integer|The number of days that are not covered by DRUG_EXPOSURE records that were used to make up the era record.|
|
||||
|
||||
### Conventions
|
||||
* Drug Eras are derived from records in the DRUG_EXPOSURE table using a standardized algorithm.
|
||||
* Each Drug Era corresponds to one or many Drug Exposures that form a continuous interval and contain the same Drug Ingredient (active compound).
|
||||
* The drug_concept_id field only contains Concepts that have the concept_class 'Ingredient'. The Ingredient is derived from the Drug Concepts in the DRUG_EXPOSURE table that are aggregated into the Drug Era record.
|
||||
* The Drug Era Start Date is the start date of the first Drug Exposure.
|
||||
* The Drug Era End Date is the end date of the last Drug Exposure. The End Date of each Drug Exposure is either taken from the field drug_exposure_end_date or, as it is typically not available, inferred using the following rules:
|
||||
* For pharmacy prescription data, the date when the drug was dispensed plus the number of days of supply are used to extrapolate the End Date for the Drug Exposure. Depending on the country-specific healthcare system, this supply information is either explicitly provided in the day_supply field or inferred from package size or similar information.
|
||||
* For Procedure Drugs, usually the drug is administered on a single date (i.e., the administration date).
|
||||
* A standard Persistence Window of 30 days (gap, slack) is permitted between two subsequent such extrapolated DRUG_EXPOSURE records to be considered to be merged into a single Drug Era.
|
||||
* The Gap Days determine how many total drug-free days are observed between all Drug Exposure events that contribute to a DRUG_ERA record. It is assumed that the drugs are "not stockpiled" by the patient, i.e. that if a new drug prescription or refill is observed (a new DRUG_EXPOSURE record is written), the remaining supply from the previous events is abandoned.
|
||||
* The difference between Persistence Window and Gap Days is that the former is the maximum drug-free time allowed between two subsequent DRUG_EXPOSURE records, while the latter is the sum of actual drug-free days for the given Drug Era under the above assumption of non-stockpiling.
|
||||
* The choice of a standard Persistence Window of 30 and the non-stockpiling assumption is arbitrary, but has been shown to deliver good results in drug-outcome estimation. Other problems, such as estimation of drug compliance, my require a different or drug-dependent Persistence Window/stockpiling assumption. Researchers are encouraged to consider creating their own Drug Eras with different parameters as Cohorts and store them in the COHORT table.
|
||||
|
||||
No.|Convention Description
|
||||
:--------|:------------------------------------
|
||||
| 1 | Drug Eras are derived from records in the DRUG_EXPOSURE table using a standardized algorithm.
|
||||
| 2 | Each Drug Era corresponds to one or many Drug Exposures that form a continuous interval and contain the same Drug Ingredient (active compound). |
|
||||
| 3 | The drug_concept_id field only contains Concepts that have the concept_class 'Ingredient'. The Ingredient is derived from the Drug Concepts in the DRUG_EXPOSURE table that are aggregated into the Drug Era record. |
|
||||
| 4 | The Drug Era Start Date is the start date of the first Drug Exposure. |
|
||||
| 5 | The Drug Era End Date is the end date of the last Drug Exposure. The End Date of each Drug Exposure is either taken from the field drug_exposure_end_date or, as it is typically not available, inferred using the following rules:<br><ul><li>For pharmacy prescription data, the date when the drug was dispensed plus the number of days of supply are used to extrapolate the End Date for the Drug Exposure. Depending on the country-specific healthcare system, this supply information is either explicitly provided in the day_supply field or inferred from package size or similar information.</li><li>For Procedure Drugs, usually the drug is administered on a single date (i.e., the administration date).</li><li> A standard Persistence Window of 30 days (gap, slack) is permitted between two subsequent such extrapolated DRUG_EXPOSURE records to be considered to be merged into a single Drug Era.</li></ul> |
|
||||
| 6 | The Gap Days determine how many total drug-free days are observed between all Drug Exposure events that contribute to a DRUG_ERA record. It is assumed that the drugs are "not stockpiled" by the patient, i.e. that if a new drug prescription or refill is observed (a new DRUG_EXPOSURE record is written), the remaining supply from the previous events is abandoned. |
|
||||
| 7 | The difference between Persistence Window and Gap Days is that the former is the maximum drug-free time allowed between two subsequent DRUG_EXPOSURE records, while the latter is the sum of actual drug-free days for the given Drug Era under the above assumption of non-stockpiling. |
|
||||
| 8 | The choice of a standard Persistence Window of 30 and the non-stockpiling assumption is arbitrary, but has been shown to deliver good results in drug-outcome estimation. Other problems, such as estimation of drug compliance, my require a different or drug-dependent Persistence Window/stockpiling assumption. Researchers are encouraged to consider creating their own Drug Eras with different parameters as Cohorts and store them in the COHORT table. |
|
||||
|
||||
\
|
||||
|
||||
Loading…
Reference in New Issue