Merge branch 'master' of https://github.com/OHDSI/CommonDataModel.wiki
commit
8b99b43ce9
|
|
@ -1,6 +1,6 @@
|
|||
<br>[The Role of the Common Data Model](TheRoleoftheCommonDataModel.md)
|
||||
<br>[Design Principles](DesignPrinciples.md)
|
||||
<br>[Data Model Conventions](DataModelConventions.md)
|
||||
<br>[The Role of the Common Data Model](The-Role-of-the-Common-Data-Model)
|
||||
<br>[Design Principles](Design-Principles)
|
||||
<br>[Data Model Conventions](Data-Model-Conventions)
|
||||
|
||||
The Observational Medical Outcomes Partnership (OMOP) was a public-private partnership established to inform the appropriate use of observational healthcare databases for studying the effects of medical products. Over the course of the 5-year project and through its community of researchers from industry, government, and academia, OMOP successfully achieved its aims to:
|
||||
|
||||
|
|
|
|||
8
Home.md
8
Home.md
|
|
@ -29,11 +29,11 @@ Welcome to the Common Data Model wiki! This wiki houses all of the documentation
|
|||
<br>[COHORT_DEFINITION](wiki/COHORT_DEFINITION)
|
||||
<br>[ATTRIBUTE_DEFINITION](wiki/ATTRIBUTE_DEFINITION)
|
||||
<br>
|
||||
<br>**Standardized Metadata**
|
||||
<br>CDM_SOURCE
|
||||
<br>**[Standardized Metadata](wiki/Standardized-Metadata)**
|
||||
<br>[CDM_SOURCE](wiki/CDM_SOURCE)
|
||||
<br>
|
||||
<br>**Standardized Clinical Data Tables**
|
||||
<br>PERSON
|
||||
<br>**[Standardized Clinical Data Tables]()**
|
||||
<br>[PERSON]
|
||||
<br>OBSERVATION_PERIOD
|
||||
<br>SPECIMEN
|
||||
<br>DEATH
|
||||
|
|
|
|||
|
|
@ -1,15 +1,15 @@
|
|||
<br>[CONCEPT](wiki/CONCEPT)
|
||||
<br>[VOCABULARY](wiki/VOCABULARY)
|
||||
<br>[DOMAIN](wiki/DOMAIN)
|
||||
<br>[CONCEPT_CLASS](wiki/CONCEPT_CLASS)
|
||||
<br>3.5 CONCEPT_RELATIONSHIP
|
||||
<br>3.6 RELATIONSHIP
|
||||
<br>3.7 CONCEPT_SYNONYM
|
||||
<br>3.8 CONCEPT_ANCESTOR
|
||||
<br>3.9 SOURCE_TO_CONCEPT_MAP
|
||||
<br>3.10 DRUG_STRENGTH
|
||||
<br>3.11 COHORT_DEFINITION
|
||||
<br>3.12 ATTRIBUTE_DEFINITION
|
||||
<br>[CONCEPT](CONCEPT)
|
||||
<br>[VOCABULARY](VOCABULARY)
|
||||
<br>[DOMAIN](DOMAIN)
|
||||
<br>[CONCEPT_CLASS](CONCEPT_CLASS)
|
||||
<br>[CONCEPT_RELATIONSHIP](CONCEPT_RELATIONSHIP)
|
||||
<br>[RELATIONSHIP](RELATIONSHIP)
|
||||
<br>[CONCEPT_SYNONYM](CONCEPT_SYNONYM)
|
||||
<br>[CONCEPT_ANCESTOR](CONCEPT_ANCESTOR)
|
||||
<br>[SOURCE_TO_CONCEPT_MAP](SOURCE_TO_CONCEPT_MAP)
|
||||
<br>[DRUG_STRENGTH](DRUG_STRENGTH)
|
||||
<br>[COHORT_DEFINITION](COHORT_DEFINITION)
|
||||
<br>[ATTRIBUTE_DEFINITION](ATTRIBUTE_DEFINITION)
|
||||
|
||||
These tables contain detailed information about the Concepts used in all of the CDM fact tables. The content of the Standardized Vocabularies tables is not generated anew by each CDM implementation. Instead, it is maintained centrally as a service to the community.
|
||||
|
||||
|
|
|
|||
|
|
@ -1 +1,20 @@
|
|||
#CDM_SOURCE
|
||||
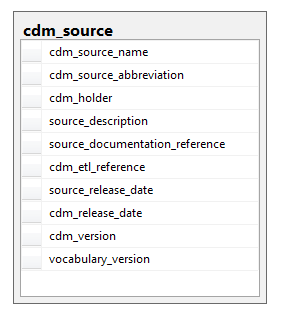
The CDM_SOURCE table contains detail about the source database and the process used to transform the data into the OMOP Common Data Model.
|
||||
|
||||
Field|Required|Type|Description
|
||||
:----|:----|:-----|:-----
|
||||
|cdm_source_name|Yes|varchar(255)|The full name of the source|
|
||||
|cdm_source_abbreviation|No|varchar(25)|An abbreviation of the name|
|
||||
|cdm_holder|No|varchar(255)|The name of the organization responsible for the development of the CDM instance|
|
||||
|source_description|No|CLOB|A description of the source data origin and purpose for collection. The description may contain a summary of the period of time that is expected to be covered by this dataset.|
|
||||
|source_documentation_reference|No|varchar(255)|URL or other external reference to location of source documentation|
|
||||
|cdm_etl _reference|No|varchar(255)|URL or other external reference to location of ETL specification documentation and ETL source code|
|
||||
|source_release_date|No|date|The date for which the source data are most current, such as the last day of data capture|
|
||||
|cdm_release_date|No|date|The date when the CDM was instantiated|
|
||||
|cdm_version|No|varchar(10)|The version of CDM used|
|
||||
|vocabulary_version|No|varchar(20)|The version of the vocabulary used|
|
||||
|
||||
## Conventions
|
||||
|
||||
* If a source database is derived from multiple data feeds, the integration of those disparate sources is expected to be documented in the ETL specifications. The source information on each of the databases can be represented as separate records in the CDM_SOURCE table.
|
||||
* Currently, there is no mechanism to link individual records in the CDM tables to their source record in the CDM_SOURCE table.
|
||||
* The version of the vocabulary can be obtained from the vocabulary_name field in the VOCABULARY table for the record where vocabulary_id='None'.
|
||||
|
|
@ -1 +1,7 @@
|
|||
# Standardized Vocabularies
|
||||
[CDM_SOURCE](CDM_SOURCE)
|
||||
|
||||
All metadata about the data should be derived from the data themselves. However, the folowing contains a few key pieces of information that are convenient especially for software applications utilizing the CDM data.
|
||||
|
||||
Below provides and entity-relationship diagram highlighting the tables withing the Standardized Metadata portion of the OMOP Common Data Model:
|
||||
|
||||
|
||||
Loading…
Reference in New Issue