1.6 KiB
1.6 KiB
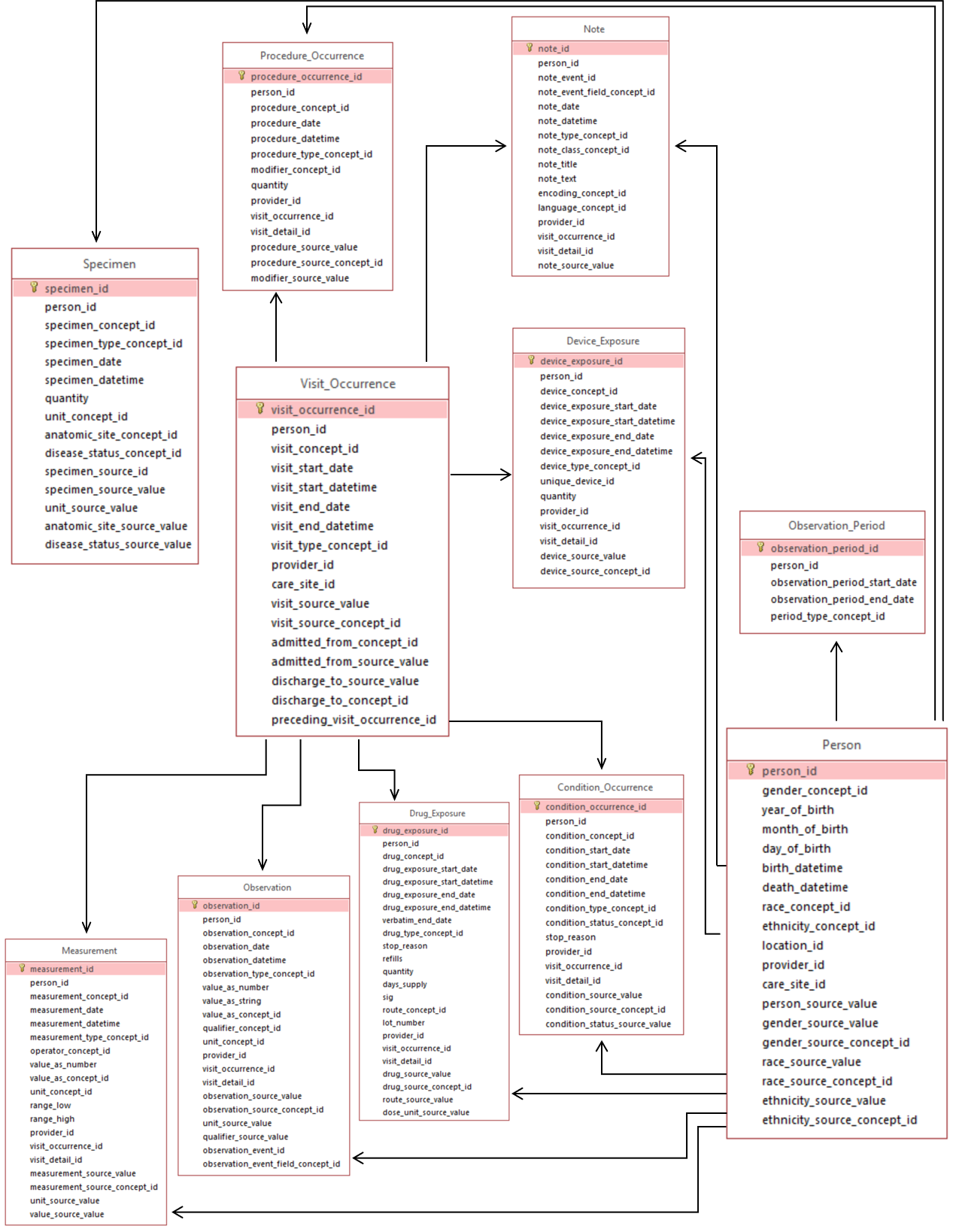
PERSON
OBSERVATION_PERIOD
DEATH
VISIT_OCCURRENCE
VISIT_DETAIL
CONDITION_OCCURRENCE
DRUG_EXPOSURE
PROCEDURE_OCCURRENCE
DEVICE_EXPOSURE
MEASUREMENT
NOTE
NOTE_NLP
SURVEY_CONDUCT
OBSERVATION
SPECIMEN
FACT_RELATIONSHIP
These tables contain the core information about the clinical events that occurred longitudinally during valid Observation Periods for each Person, as well as demographic information for the Person. Below provides an entity-relationship diagram highlighting the tables within the Standardized Clinical Data portion of the OMOP Common Data Model: